NeuroNex News
August 2024, Issue #7
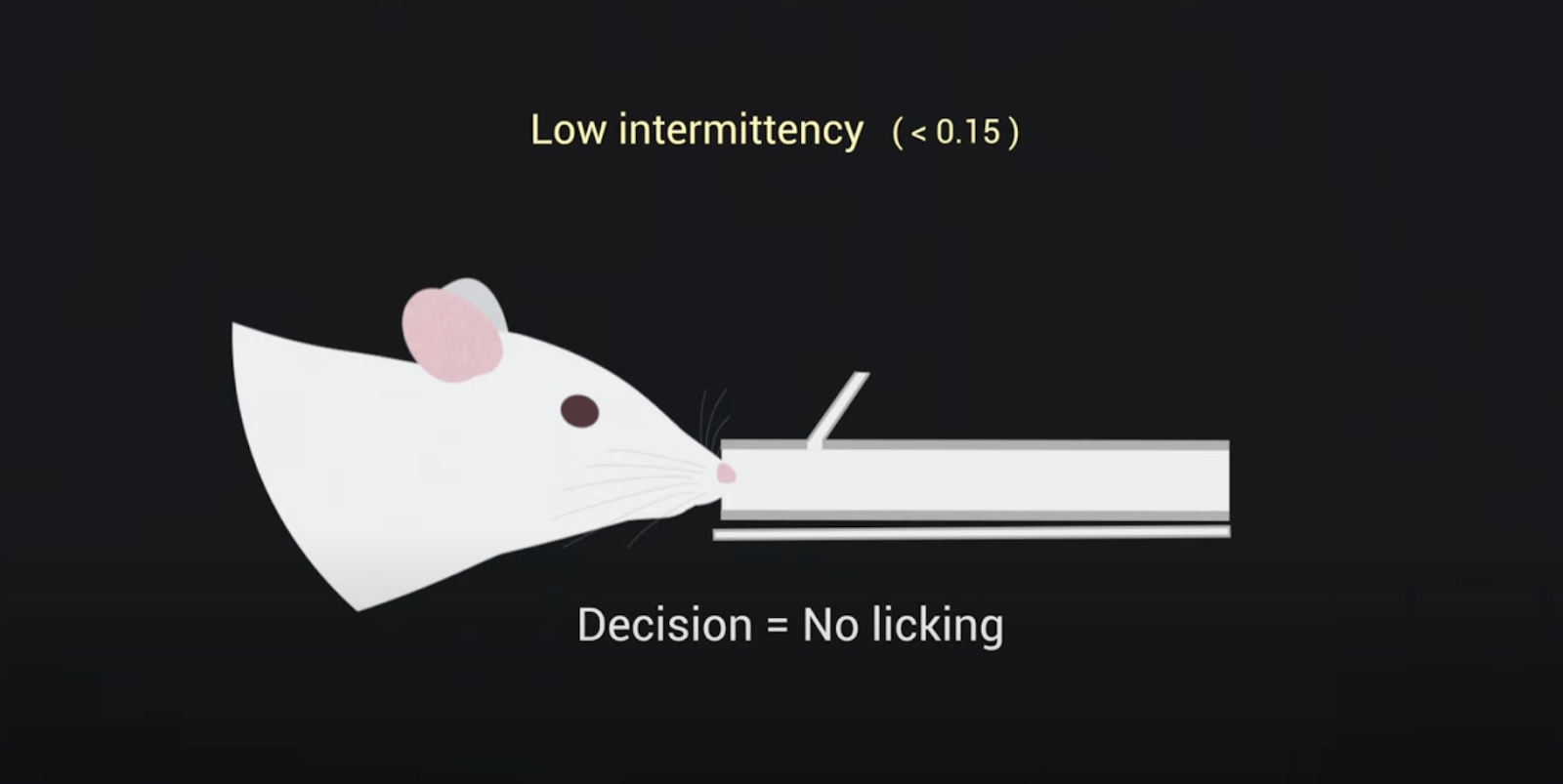
In a recent study on odor intermittency, researchers investigated how mice perceive and track odors as the intensity and frequency of detectable scents fluctuate. Watch a video that highlights these findings.
Animals depend on their sense of smell to navigate a world rich with various odors. These odors often create turbulent airborne trails called plumes, which result in intermittent whiffs of scent. Odor plumes have characteristics that change the further you move from the source of the smell.
In a recent study, researchers with the Odor2Action network focused on “odor intermittency,” which refers to the percentage of time that a smell is detectable above a certain level at a specific location within the scent plume. The closer you are to the source, the higher the percentage of time you will detect the odor. Researchers wanted to investigate if mice could use these changes in intermittency to locate an odor source.
“While it is not well understood how mammals navigate dynamic odor plumes in their environment, neuro-behavioral olfactory research increasingly indicates that the sense of smell is a “fast” sense,” says Justus Verhagen, author of the study and co-PI on the Odor2Action network.
The researchers trained mice to distinguish between plumes with different levels of intermittency. The results showed that mice could differentiate between plumes with high and low intermittency. The activity in the mice’s brains, specifically in the olfactory bulb, where the brain processes smell, reflected their ability to do this.
The study also found that how mice sniff, which changes a lot during odor-guided navigation, affects both their performance in identifying intermittency and the brain's representation of intermittency. Overall, this research highlights that intermittency is a key feature animals use to navigate by smell. By understanding how animals process these intermittent odor signals, we can gain better insights into how they use scent to navigate their environment.
Watch a video that highlights these findings.

Mona Marie works on cloning a Class I smell receptor in an effort to understand how these receptors work in mammals. Photo credit: Ichie Ojiro
Mona Marie is a postdoctoral associate at Duke University’s Department of Molecular Genetics & Microbiology. She works with Hiroaki Matsunami, Co-PI on the NeuroNex Project, Odor2Action: Discovering Principles of Olfactory-Guided Natural Behavior. The team is focused on understanding the molecular mechanisms behind taste and smell in mammals. As part of that effort, Marie relies on a combination of lab experiments and computational analysis to study how smell or odorant receptors work. She uses lab techniques such as molecular docking and cell culture systems to discover chemicals that either trigger or block two types of odorant receptors–Class I receptors, which are sensitive to fatty acids, and Class II receptors, which respond to a broader range of odors.
Marie’s work has resulted in a collection of chemicals that impact these odorant receptors. She investigates how these chemicals activate the receptors and how different mixtures influence receptor activity, creating models to predict their effects.
Marie has shared these chemical libraries with others in the NeuroNex network, including Matt Wachowiak, Professor of Neurobiology, and Madison Herrboldt, a postdoctoral research associate—both at the University of Utah. The Wachowiak lab uses transgenic mice to study how these receptors work in live organisms—information that helps Marie and others on the team better understand the signals involved in smell. “The insights gained from these studies are instrumental in hypothesizing a potential consensus code for how Class I and Class II odorant receptors tuning is modulated in vivo, bringing us closer to unraveling the intricacies of the olfactory system,” says Marie.
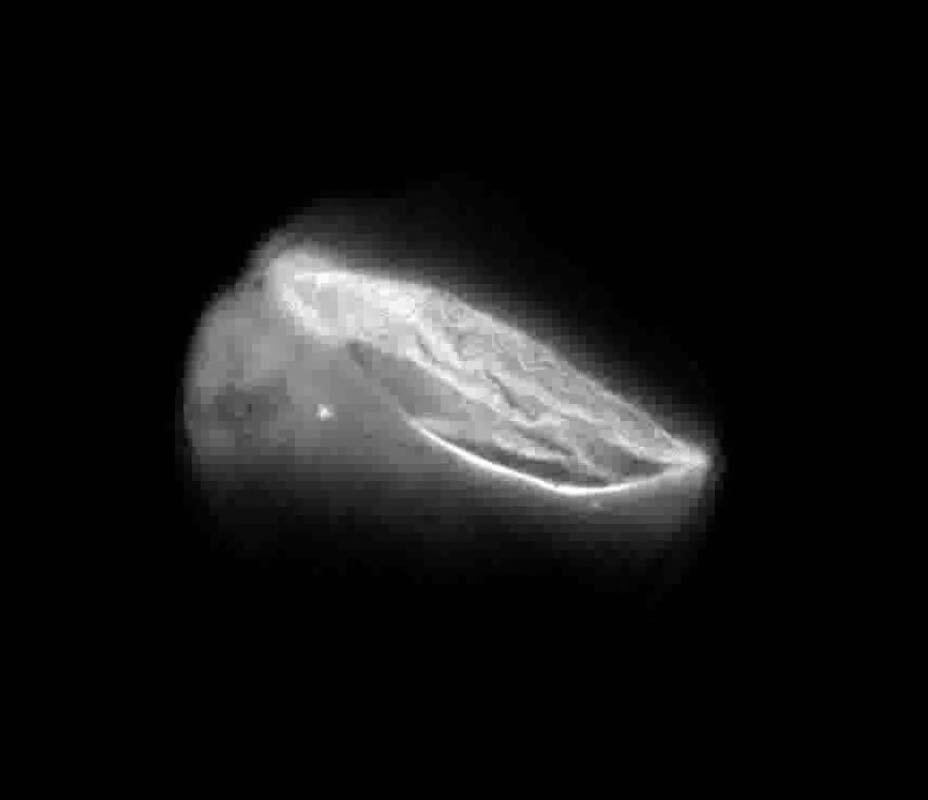
Here, bioluminescence is observed in a mouse’s choroid plexus, a network of cells and blood vessels in the brain’s ventricles. Researchers with the NeuroNex project, Bioluminescence for Optimal Brain Control and Imaging, did this by injecting a water-soluble coelenterazine (CTZ) directly into the brain ventricle of an anesthetized transgenic mouse. Credit: Eric Klein and Manuel Gomez-Ramirez
The NeuroNex Project, Bioluminescence for Optimal Brain Control and Imaging, recently published several papers in the “Special Section on Molecular NeuroPhotonics” in the journal Neurophotonics.
The collection of papers explores significant advancements in bioluminescence technologies and their applications. They cover the development of novel imaging techniques for real-time detection of bioluminescent signals, enhancing the precision of biological monitoring. Innovations in bioluminescent reporter systems are discussed, highlighting improvements in sensitivity and versatility for tracking gene expression and cellular activities. The papers also examine the use of bioluminescence to study protein interactions and cellular dynamics, providing insights into molecular processes. Additionally, they review practical applications of bioluminescence in drug discovery, environmental monitoring, and medical diagnostics, and suggest future research directions to further enhance these technologies. Overall, these studies demonstrate the expanding role of bioluminescence in various scientific and medical fields.
Papers produced by the group and published in the special section are as follows:
- Engineering luminopsins with improved coupling efficiencies
- Efficient opto- and chemogenetic control in a single molecule driven by FRET-modified bioluminescence
- Toward a brighter constellation: multiorgan neuroimaging of neural and vascular dynamics in the spinal cord and brain
- BioLuminescent OptoGenetics in the choroid plexus: integrated opto- and chemogenetic control in vivo
